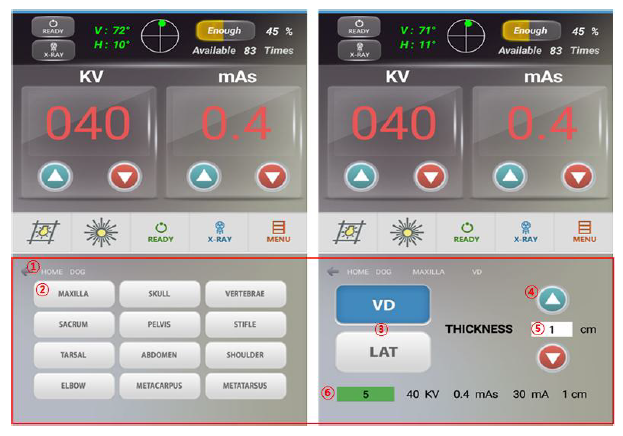
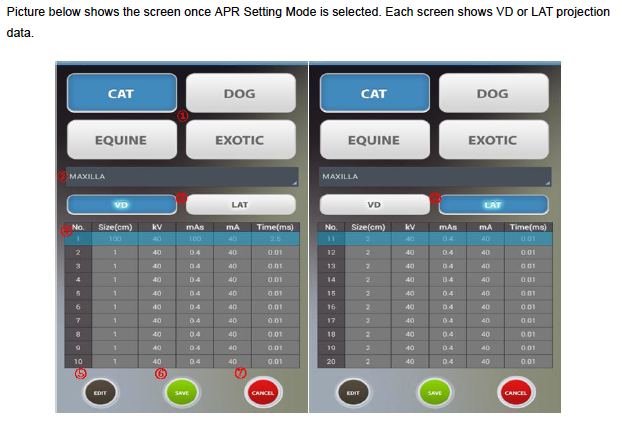
Description
Ecotron EPX-F1600B Portable X-Ray Generator with Battery
Portable X-RAY system Ecotron’s family of high frequency portable x-ray systems offers the highest power- to- weight rations available. Our EPX series to the next level providing an optimum mix of power, Ultra light, Compact size, Wireless/Wire PC interface. Wireless ;Wire Hand & Foot switch. Ready for DR system and Collimator with an enclosed high hardness acrylic mask and dual laser pointer which can help center position clearly displayed even in a day time.
Our radiology systems offer high frequency portable radiography in an economical combination with table for in-clinic and out-clinic use. Equine, mixed, small animal and zoo practices can put EPX Models to daily use with conventional x-ray film or digital imaging systems for excellent radiographic results. EPX portables features automatic and dynamic line voltage compensation to assure you of consistent radiation output over a range of available electrical conditions. EPX feature low voltage input operation compared with competitors so you will always get the maximum possible output even with low input voltages. Small Xray tube focal sizes produce images with
excellent fine detail resolution.
Continually adjustable light beam collimators and dual, adjustable integrated dual laser pointer are standard on each model, elimination guesswork in positioning. Technique memory is also standard on all models. EPX por1able units come with carrying case to protect your investment.
The EFX-F1600B is compact battery operated portable x-ray generator suitable for veterinary mobile use, weighing in at only 8.2kG and with 90kV and 20mA range puts it ahead of most of the competition in this weigh range.
Key Features:
– Provide high resolution imaging at lower dosages.
– Durable design with solid body.
– Touch screen console
– Calibrated cassette size indicator dials
– Available with Inverted control panel for table use.
– Two stage dynamic, Auto line compensation.
– Easy access to DR systems.
– Collimator with High Lux LED light and Dual Integrated Laser Pointers
– Wired handswitch
– Battery with Super Capacitor Technology
Ecotron EPX-F1600B Portable X-Ray Generator with Battery – Specifications:
Output Power 1.6kW
Battery Charger Input Power Voltage: 220V
Battery voltage: 29.4V
Phase & Frequency: Single / 50 / 60 Hz
Radiography kV Range: 40kV ~ 90kV
mAs Range: 0.4mAs – 32mAs
mA Range:10~16mA
Max.kV Deviation: 3%
Max.mAs Deviation: 5%
X-ray Tube:
Model Name D-0814 TOSHIBA
Focal Spot 0.8mm x 0.8mm
Target Angle 16 degree
Anode Heat Storage 7 kHU
Collimator with Laser Pointer:
Type: Double slit type, Manually operation
Min.X-ray Field Size: 0cm x 0cm @1m SID
Max X-ray Field Size: 43cm x 43cm @ 75cm SID
Lamp: 3V 56.5W LED
Dimension: 369mmx203mmx222mm (handle and trigger included)
Packing Size: 520mmx400mmx330mm
Net-weight:8.2kg
Tube voltage current mAs table
40KV ~ 70KV 20mA 0.4 ~ 5 mAs
40KV ~ 70KV10mA6.4 ~ 32 mAs
71KV ~ 80KV20mA0.4 ~ 5 mAs
71KV ~ 80KV10mA6~32 mAs
81KV ~ 90KV15mA0.4 ~ 5 mAs
81KV ~ 90KV10mA16~32 mAs
1) SAFETY
● EN 60601-1:2006/A1:2013
● EN60601-1-3:2008
● EN60601-1-6:2010
● EN62366:2008
● EN60601-2-28:2010
● EN60601-2-54:2009
2) EMC
● EN60601-1-2:2015
The BATTERY PORTABLE EPX- B SERIES X-ray system with CE marking comply with the European Council Directive concerning Medical Devices. One of the harmonized standards of this Directive defines the permitted levels of electromagnetic emission from this equipment and its required immunity from the electromagnetic emission of other devise.
It is not possible, however to exclude with absolute certainty the possibility that other high frequency electronic equipment, which is fully compliant to the EMC regulations, will not adverse effect the operation of this x-ray system. If the other equipment has a comparatively high level of transmission power and is in close proximity to the generator, these EMC concerns (the risk of interference) may as mobile telephones, cordless microphones and other similar mobile radio equipment be restricted from the vicinity of this X-ray system.
3) OTHERS
● EN ISO 13485:2016 Medical devices – Quality management systems – Requirements for regulatory purposes
● EN ISO 14971:2012 Medical devices – Application of risk management to medical devices (ISO 14971:2007, Corrected version 2007-10-01)
● EN980:2008 Symbols for use in the labeling of medical devices
● EN1041:2008 Information supplied by the manufacturer of medical devices
● EN62304:2006 Medical device software – Software life-cycle processes IEC 62304:2006
● MEDDEV 2.12.1/Rev.8 Medical Devices Vigilance System
● MEDDEV 2.12.2/Rev.2 Post Market Clinical Follow-up studies
4) CLINICAL EVALUATION
● MEDDEV 2.7.1/Rev.4 Clinical evaluation: Guide for manufacturers and notified bodies
Additional information
| Weight | 20 kg |
|---|